Do you remember when you learned about mitochondria as the ‘powerhouse of the cell’? I do. It felt like a biology class throwaway line—important, but distant. Years later, sitting with my father during his chemo sessions, I began to wonder: what if those little powerhouses decided the fate of the entire building? That uncomfortable question—merged with late-night dives into YouTube lectures and stacks of biology papers—sparked this exploration of what cancer might be, what we may have gotten wrong, and why lifestyle isn’t just a buzzword. Brace yourself: We’re about to turn cancer research inside out, straight into the crux of your own cell’s engine room.
1. Setting the Stage: Why the Genetic Model Isn’t Everything
If you’ve followed cancer research for any length of time, you know that the genetic model dominates the conversation. For decades, the prevailing belief has been that cancer is primarily a genetic disease—mutations in your DNA spark abnormal cell growth, and that’s the end of the story. But what if that’s not the whole picture? According to Thomas Seyfried, a leading voice in Cancer Management and Cancer Prevention Strategies, the evidence points in a different direction: cancer as a metabolic disorder (0.29-0.38).
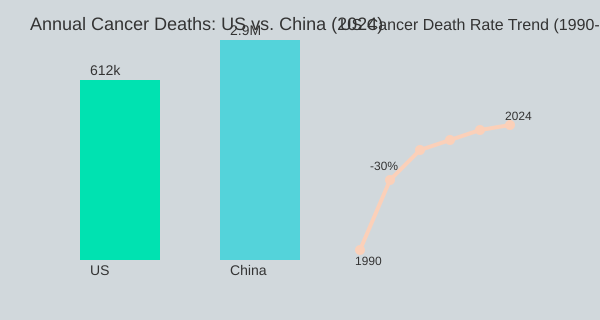
Let’s pause and look at the numbers. Despite billions poured into genetic research, Cancer Statistics remain grim. In the United States alone, nearly 2 million new cancer diagnoses are made each year, and about 1,700 people die from cancer every single day (3.01-3.13). That’s roughly 70 deaths per hour. In China, the numbers are even more staggering, with 8,000 cancer deaths daily (3.19-3.26). These aren’t just numbers—they represent lives, families, and communities affected by a disease that, research shows, is not going away. In fact, projections suggest the situation will only worsen by 2050 (3.37-3.42).
So, what’s missing? Seyfried argues that the focus on genetics has led us to overlook the powerful role of Lifestyle Factors Cancer. He points to striking patterns: cancer was once extremely rare among African tribes living traditionally, but rates soared when Western lifestyles—think processed foods, sedentary habits, and environmental toxins—became the norm (0.46-0.56). This isn’t just a human story. Consider this:
“Wolves in the wild don’t die from cancer but cancer is the number one killer of domestic dogs. Why? Lifestyle issues.” (0.58-1.07)
This unexpected case study highlights a crucial point. In wild environments, where animals follow their natural diets and activity patterns, cancer is rare. But domestication—marked by processed foods and less movement—brings a surge in cancer rates. The same pattern emerges in people. As societies modernize, cancer becomes more common (0.48-0.56).
Despite the dominance of the genetic theory, Cancer Statistics show no major advances in reducing death rates (0.22-0.24, 3.53-4.03). Lung cancer remains the leading cause of cancer death for both men and women in the US, with pancreatic, colon, and breast cancers also on the rise (4.30-4.49). The numbers don’t lie: cancer is a global epidemic, and the current approach isn’t working.
Seyfried’s research, and the broader Thomas Seyfried Cancer Research movement, challenges us to rethink everything we know about cancer. He insists:
“Cancer is very preventable when the medical establishment acknowledges what I know about this disorder.”
Why does this matter for you? Because understanding the metabolic roots of cancer opens new doors for Cancer Prevention Strategies. It means looking beyond genes to the choices we make every day—what we eat, how we move, and the environments we create. The evidence is mounting: lifestyle changes can influence cancer risk as much, if not more, than genetics alone.
US
620K
China
2.9M
Pancreas
+25%
Colon
+15%
Annual Cancer Deaths & Incidence Trends
0
1M
2M
3M
US Annual Deaths
China Annual Deaths
Pancreatic Cancer Incidence (10yr)
Colon Cancer Incidence (10yr)
2. Mitochondrial Mayhem: How Faulty Powerhouses Spark Disease
When you think about cancer, you might picture hundreds of different diseases—lung, colon, breast, brain, and more. Under the microscope, each type looks unique, with its own set of genetic mutations and cellular oddities (7.15-7.35). But if you dig deeper, research shows there’s a surprising common thread: mitochondrial dysfunction sits at the heart of cancer’s development, uniting these diverse diseases under the Metabolic Theory of Cancer.
The Metabolic Theory of Cancer: A New Perspective
Traditional cancer theories focus on genetic mutations. However, the Metabolic Theory of Cancer suggests that the real culprit is how your cells generate energy. In healthy cells, mitochondria—your body’s powerhouses—efficiently burn fuel using oxygen. But in cancer cells, these mitochondria become faulty, forcing cells to fall back on a primitive process: fermentation (7.37-7.55).
The Warburg Hypothesis: Cancer’s Ancient Energy Pathway
This idea isn’t new. Otto Warburg, a Nobel Prize-winning scientist, first noticed that cancer cells prefer to make energy without oxygen, even when oxygen is available. This phenomenon, known as the Warburg Effect, means that cancer cells rely on fermentation—a throwback to ancient life on Earth, when oxygen was scarce (11.02-11.23). Instead of using oxygen to fully burn glucose, cancer cells use glucose and glutamine to generate energy quickly, but inefficiently (9.16-9.32).
Glucose Metabolism and Glutamine Fermentation: Cancer’s Dual Fuel System
So, what fuels this fermentation? Two main sources: glucose (sugar) and glutamine (an abundant amino acid in your bloodstream). When your body is deprived of oxygen—even for a short time—cells switch to burning these fuels, producing lactic acid (from glucose) and succinic acid (from glutamine) as waste (9.43-9.55). In cancer, this process is always “on,” regardless of how much oxygen is present (10.44-10.49).
Why Cyanide Kills Normal Cells—But Not Tumors
Here’s a striking example: cyanide is deadly because it blocks your cells from using oxygen. For most of your body, that’s a death sentence within minutes (10.13-10.23). But cancer cells? They can survive, even thrive, in the presence of cyanide, because they’re already relying on fermentation, not oxygen (10.30-10.35). As Dr. Thomas Seyfried puts it,
“The evidence is massive to support that cancer is a metabolic disorder.”
Different Cancers, Same Metabolic Defect
Despite their varied appearances and genetic profiles, all major cancers—lung, colon, breast, brain—share this metabolic defect. They depend on fermentation for survival and growth (7.58-8.11). This insight is shifting the focus of Metabolic Therapy Cancer research, moving from targeting genes to targeting cancer’s energy supply.
| Cancer Type | Main Energy Pathway | Key Fuels | Waste Products | Response to Cyanide |
|---|---|---|---|---|
| Lung, Colon, Breast, Brain | Fermentation | Glucose, Glutamine | Lactic Acid (glucose), Succinic Acid (glutamine) | Survive (unlike normal cells) |
Understanding mitochondrial dysfunction cancer and the metabolic commonality among tumors opens new doors for prevention and treatment. By targeting glucose metabolism and glutamine fermentation, metabolic therapies may offer hope where traditional approaches fall short. The science is clear: at its core, cancer is a disease of broken powerhouses, and fixing the cell’s energy engine could be the key to stopping cancer in its tracks.
3. The Old Debate: Warburg, Seyfried, and the Roots of a Theory
Let’s take a quick trip back to the 1920s, when a German scientist named Otto Warburg was quietly shaking the foundations of cancer research. If you’ve ever wondered where the Warburg Hypothesis comes from, it’s this era—an age before gene sequencing, before molecular biology, when scientists were still piecing together the basics of cellular life (12.21-12.44).
Imagine yourself as a single cell, living in a world with barely any oxygen. You’re hustling for sugar, fermenting it for energy, because that’s all you know. Warburg noticed something odd: even when cancer cells had plenty of oxygen, they still chose to ferment glucose, just like those ancient, pre-oxygen cells. This oddity became known as the Warburg Effect—the idea that cancer cells prefer fermentation over respiration, even when oxygen is available (12.38-12.44).
Warburg’s hypothesis was bold: he believed that faulty cell respiration—essentially, broken mitochondria—was the root cause of cancer. This was a radical departure from the idea that cancer was simply a genetic disease. For decades, though, the genetic theory took center stage, and Warburg’s ideas faded into the background. But as research shows, the Metabolic Theory of Cancer is making a comeback, with scientists like Thomas Seyfried leading the charge.
Now, here’s where things get personal and practical. Fast forward to the 1990s at Case Western Reserve University. Linda Nebling, a PhD nursing student, decided to put Warburg’s theory to the test. She worked with two children diagnosed with aggressive brain cancer—cases so severe they were considered “hopeless.” Inspired by the Warburg Hypothesis, Nebling introduced a ketogenic diet to these patients, aiming to lower their blood sugar and starve the cancer cells of their favorite fuel (13.01-13.32).
The results? One child survived longer than expected, and the other was lost to follow-up. While not a miracle cure, it was a glimmer of hope—a real-world example of how a metabolic approach could make a difference. Clinical evidence is increasingly supporting metabolic strategies like the Ketogenic Diet Cancer approach, especially for hard-to-treat cases.
But there’s more to the story. Recent research, including Thomas Seyfried Cancer Research, has uncovered that cancer cells don’t just crave glucose—they’re also addicted to glutamine. This “one-two punch” of glucose and glutamine fuels cancer’s relentless growth. Targeting both, through diet or other metabolic therapies, is now seen as a promising direction for future treatments.
For a long time, the genetic theory of cancer hijacked the narrative. But as Seyfried himself admits, he started out believing in the genetic model, only to be swayed by mounting evidence for the metabolic theory. As he puts it:
“All cancers are a singular type of disease. It’s just that they happen in different tissues…but they’re all very, very similar.”
Today, the Warburg Effect is re-emerging in therapeutic thinking, and a metabolic approach shows real-world clinical promise. The story is far from over, but the roots of this theory are deeper—and quirkier—than you might expect.
| Year | Key Event |
|---|---|
| 1920s | Warburg formulates hypothesis on cancer metabolism |
| 1990s | Case Western: Two brain cancer ‘hopeless cases’ tried ketogenic diet intervention based on Warburg’s ideas |
4. What the Statistics Say: Cancer by the Numbers
When you look at Cancer Statistics today, the numbers are staggering—and they’re not just numbers. They represent real lives and families impacted every single hour. According to the American Cancer Society, nearly 2 million new cancer cases are expected to be diagnosed in the United States in 2024 (2.54-3.04). Even more sobering: about 612,000 Americans will lose their lives to cancer this year. That’s roughly 1,700 deaths every day, or 70 people every hour (3.06-3.19).
If you zoom out globally, the picture becomes even more daunting. In China, for example, about 8,000 people die from cancer every day (3.19-3.26). The population is much larger, but the scale of the challenge is clear. While specific numbers for the UK and other countries vary, the trend is similar: cancer is a growing global epidemic, and projections suggest it will only get worse by 2050 (3.30-3.42).
Cancer Prevention Strategies: The Real Impact of Anti-Smoking Campaigns
You might have heard that cancer death rates in the U.S. have dropped by about 30% since the 1990s. On the surface, this sounds like a huge victory for Cancer Management. But when you dig deeper, the story is more nuanced. The anti-smoking campaigns of the 1990s played a massive role in this decline (5.50-6.12). As one expert put it:
“If we didn’t stop smoking in the 90s…the trajectory would be very, very high. Because we stopped smoking…we have 33% lower death than if we didn’t stop smoking.”
So, this drop is less about breakthrough treatments and more about Cancer Prevention Strategies—specifically, fewer people smoking. If smoking rates hadn’t fallen, the death rate would have climbed even higher. The trajectory is still rising, just not as steeply as it could have (6.12-6.36).
Which Cancers Are Most Common—and How Are They Changing?
Lung cancer has long been the deadliest cancer for both men and women in the U.S. (4.34-4.40). But the landscape is shifting. Today, colon cancer and pancreatic cancer are both on the rise, even as smoking-related lung cancer rates decline (4.43-4.49). This suggests that while lifestyle changes like quitting smoking have helped, other Lifestyle Factors—such as diet, exercise, and perhaps even environmental exposures—are driving up other cancer types.
The overall number of new cancer cases seems to increase every year, making it a moving target for researchers and policymakers (5.04-5.13). Despite the progress in prevention, especially through anti-smoking efforts, the overall burden of cancer continues to grow. This highlights the urgent need to focus not just on treatment, but on comprehensive prevention and management strategies that address all risk factors.
Digesting the ‘30% Drop’: What’s the Real Story?
It’s tempting to celebrate a 30% reduction in cancer deaths, but remember: most of this improvement comes from prevention, not new therapies. The fight against cancer is far from over. As research shows, certain cancers like colon and pancreatic are increasing despite preventive improvements. This underscores the importance of ongoing research into Lifestyle Factors Cancer and the need for innovative Cancer Management approaches.
5. Metabolic Therapy: Hope, Hype, and the Everyday Human
When you hear the term Metabolic Therapy Cancer, it might sound technical, but the core idea is surprisingly simple: cut off cancer’s fuel supply. Imagine your body’s cells as tiny engines. Cancer cells, in particular, are like gas-guzzling cars—they thrive on certain fuels, especially glucose. By changing what you eat, you can potentially starve these cells, making it harder for them to grow and spread. That’s the heart of metabolic therapy (1.08-1.10).
What Is Metabolic Therapy in Plain English?
Metabolic therapy is about using diet and lifestyle to change the fuels available to your cells. The goal? To tip the balance so cancer cells struggle to survive, while your healthy cells keep running strong. This approach is gaining attention because it’s both a cancer prevention strategy and a possible treatment (1.12-1.13).
“With metabolic therapy you can use it as both a prevention and a treatment…you can actually reduce risk for cancer, you can take away the fear.” (1.20-1.24)
Ketogenic Diet Cancer: Science and Real-World Stories
The ketogenic diet is a popular form of metabolic therapy. By cutting carbohydrates, you force your body to burn fat for energy. This means less glucose is available for cancer cells, which often rely on sugar to grow. Research shows that some patients, even those with advanced or terminal cancer, have outlived their initial prognosis after switching to a ketogenic diet (1.15-1.17). While these stories are anecdotal, they’re becoming more common, and emerging studies are starting to back them up.
Why does this work? It goes back to the Warburg Hypothesis—a theory dating back to Otto Warburg, who found that cancer cells prefer to ferment glucose for energy, even when oxygen is present (13.30-13.35). By restricting glucose, you’re essentially turning off one of cancer’s favorite energy sources.
Caloric Restriction Therapy: Can Eating Less Really Help?
Another approach is caloric restriction therapy. This doesn’t just mean skipping dessert; it’s about consistently eating fewer calories to limit the energy available to all cells, including cancer. Studies indicate that caloric restriction can slow tumor growth and, in some cases, make traditional treatments more effective. It’s not a magic bullet, but it’s a tool that’s gaining traction in both research and real-world practice.
Mixing Therapy with Prevention: Does It Matter If You Aren’t Sick Yet?
You might wonder if these strategies are only for those already diagnosed. The answer is a resounding yes—prevention matters. Many people have lived for years without knowing they were at risk (1.07-1.08). By adopting metabolic therapy early, you can potentially reduce your risk and, as the quote above suggests, “take away the fear.”
Terminal Cancer Patients and Unexpected Remissions
One of the most intriguing aspects of metabolic therapy is the growing number of terminal cancer patients who are outliving their prognosis (1.13-1.17). While not every story ends in remission, these cases are fueling hope and driving more research into metabolic interventions as both a treatment and a preventive measure.
Practical Wild Card: What Are You Eating Today?
Think of your cells as having tiny fuel gauges. Every meal you eat nudges those gauges one way or another. Are you filling up on foods that feed cancer, or are you choosing options that support your body’s defenses? The science is still evolving, but the trend is clear: what you eat matters—sometimes more than you think.
6. Beyond the Headlines: Why Lifestyle Matters More Than a DNA Report
When you think about cancer prevention, it’s easy to focus on genetics or the latest headlines about environmental carcinogens. But research shows that lifestyle factors play a much bigger role in cancer risk than most people realize. In fact, your daily habits—what you eat, how you move, and the environment you create inside your body—can tip the scales far more than a DNA report ever could.
Modern Lifestyles: The Hidden Risk Factor
Let’s start with a striking observation from global health studies. Cancer was extremely rare in traditional African tribes living according to their ancestral ways (0.46-0.50). But as soon as Western lifestyle habits—processed foods, sedentary routines, and urban stress—entered the picture, cancer rates began to rise (0.52-0.54). This isn’t just an isolated trend. Similar patterns have been documented in other societies after ‘modernization.’ The message? Lifestyle factors and cancer are tightly linked, often in ways that slip under our radar.
The Forgotten Impact of Diet: Glucose and Glutamine
Most people know that sugar isn’t great for health, but few realize how much high-glucose and high-glutamine diets can fuel cancer’s growth. According to the metabolic theory of cancer, cancer cells thrive on glucose and glutamine, using these nutrients to power their rapid division. Studies indicate that when you consistently eat foods high in sugar and processed carbohydrates, you’re creating an internal environment that encourages cancer cells to flourish. It’s not just about calories or weight—it’s about the metabolic signals you’re sending to your cells every day.
Exercise: The Underrated Cancer Prevention Tool
Exercise is often promoted for heart health or weight loss, but its role in cancer prevention strategies is just as important. Regular physical activity improves mitochondrial function, reduces inflammation, and helps regulate blood sugar—all factors that make it harder for cancer cells to gain a foothold. Research shows that even moderate exercise can lower your risk for several types of cancer, making it a powerful (and often overlooked) tool in your prevention toolkit.
Environmental Carcinogens vs. Internal Cellular Environment
There’s no denying that environmental carcinogens—like tobacco smoke, certain chemicals, and pollutants—are dangerous. But here’s where it gets interesting: the health of your cells’ internal environment may matter even more. If your metabolic health is strong, your cells are better equipped to repair damage and resist transformation into cancer cells. On the other hand, a poor diet and lack of exercise can weaken these defenses, making you more vulnerable even if you avoid obvious toxins (4.49-4.57).
How Do Your Habits Rate? (No Grading, Promise!)
Take a moment to reflect: How often do you eat processed foods? How much do you move each day? Are you exposed to unnecessary chemicals at home or work? These questions aren’t about guilt—they’re about awareness. As one expert put it,
“A lot of us are doing things without the knowledge that it would put us at risk.”
By understanding the real impact of your lifestyle choices, you can take meaningful steps to reduce your cancer risk—no genetic test required.
In the end, the evidence is clear: changing daily habits provides measurable risk reduction, even without genetic interventions. The power to shape your health is, quite literally, in your hands.
7. The Great Unlearning: How Changing Our Minds Changes Outcomes
Have you ever found yourself clinging to an old belief, even when new evidence nudges you to reconsider? If so, you’re not alone. The way we think about cancer—its origins, its prevention, and its treatment—has been shaped for decades by the genetic model. But what happens when a new idea, like the Metabolic Theory of Cancer, challenges everything you thought you knew? This is where the great unlearning begins, and it’s as much about personal growth as it is about medical progress.
Cultural resistance to new cancer models isn’t so different from the hesitation you might feel when breaking an old habit. Think about a time when you changed your mind about something big—maybe a career, a relationship, or a deeply held opinion. At first, it’s uncomfortable. You might even feel defensive. But as you let go, you make room for new possibilities. In medicine, this process is happening right now, as more people question whether cancer is purely a genetic disease or if, as the Metabolic Theory of Cancer suggests, it’s rooted in how our cells process energy.
Research shows that this shift doesn’t erase the value of genetic insights. Instead, it reframes the cancer battle. The metabolic perspective, championed by researchers like Thomas Seyfried and inspired by Otto Warburg’s early work, argues that cancer cells rely on fermentation for energy due to mitochondrial dysfunction. This isn’t just a technical distinction—it’s a call to rethink Cancer Prevention Strategies and therapy. If cancer’s roots are metabolic, then you have more influence than you might think.
Why is ‘metabolism’ such a hopeful word in this context? Because it’s something you can change. Unlike your genes, your metabolism responds to what you eat, how you move, and even how you manage stress. Studies indicate that lifestyle factors—diet, exercise, and caloric restriction—can play a significant role in cancer risk and prevention. The idea that prevention is as much about breakfast as about biology isn’t just catchy; it’s rooted in science. Metabolic therapies, including ketogenic diets and targeted caloric restriction, are being explored as ways to starve cancer cells of the fuel they crave, offering new hope for both prevention and treatment (see research from Seyfried and others).
But here’s the challenge: adopting the metabolic theory means accepting a new level of control and responsibility. For many, that’s empowering. For others, it’s daunting. The medical field, too, has been slow to shift its focus from genes to metabolism. Yet, as public knowledge grows, so does the momentum for change. The more you understand about metabolic health, the more you can advocate for yourself and your loved ones.
“When we understand what’s causing it and what we’re not doing to prevent or treat it, it’ll be recognized as the singular greatest tragedy in the history of medicine worldwide.”
(Transcript 4.10-4.16)
This quote underscores the urgency of unlearning old models and embracing new ones. The tragedy isn’t just in the disease itself, but in our reluctance to change how we think about it. As you explore Metabolic Therapy Cancer approaches, remember: the power to influence outcomes may be closer than you realize. Sometimes, the most profound changes begin with a willingness to rethink what you thought you knew.
8. FAQ: Unwrapping the Metabolic Mystery (and Misconceptions)
As you explore the Metabolic Theory of Cancer, it’s natural to have questions—especially when this approach challenges decades of mainstream thinking. Let’s address some of the most common curiosities and misconceptions, drawing from Professor Thomas Seyfried’s research and the latest science.
Is cancer really preventable by lifestyle changes?
Research shows that many cancers are influenced by lifestyle factors. Seyfried emphasizes that cancer was once rare among populations living traditional lifestyles—think indigenous tribes or wild animals—where diets were low in processed carbohydrates and physical activity was high. Today, with rising cancer rates worldwide, the evidence points to processed foods, sedentary habits, chronic stress, and environmental toxins as key contributors. While not every cancer case is preventable, adopting Cancer Prevention Strategies—like regular exercise, fasting, and reducing sugar intake—can significantly lower your risk by supporting healthy mitochondrial function.
Does the ketogenic diet work for all cancer types?
The Ketogenic Diet Cancer approach is promising, but it’s not a universal solution. Seyfried’s research and case studies show that many tumors, especially those with high glucose dependence, may respond well to ketogenic diets or caloric restriction. However, not all cancers are identical. Some may adapt or use alternative fuels, like glutamine. Clinical trials are ongoing, and while the ketogenic diet is generally safe, it’s best to work with a knowledgeable healthcare provider to tailor any metabolic therapy to your specific needs.
What’s the difference between the metabolic and genetic cancer theories?
The mainstream view holds that cancer is driven by genetic mutations. In contrast, the Metabolic Theory of Cancer argues that mitochondrial dysfunction—disrupting how cells produce energy—comes first. Seyfried and others point to experiments where transferring healthy mitochondria into cancerous cells can restore normal function, while placing a healthy nucleus into a cancerous cytoplasm (with damaged mitochondria) leads to uncontrolled growth. This suggests that metabolism, not just genes, is at the heart of cancer’s origins.
Are there risks to caloric restriction or metabolic therapies?
Like any intervention, metabolic therapies aren’t risk-free. Caloric restriction or strict ketogenic diets can cause fatigue, nutrient deficiencies, or unintended weight loss if not managed carefully. It’s important to monitor your health, ideally with medical supervision, and to use tools like the Glucose Ketone Index (GKI) to track your metabolic state. Remember, what works for one person may not suit another—personalization is key.
How can I know if I’m at risk?
While some risk factors—like inherited mutations—are beyond your control, most people can influence their risk through lifestyle choices. Monitoring blood sugar, maintaining a healthy weight, staying active, and minimizing exposure to known carcinogens are practical steps. Tools like the GKI calculator offer a way to quantify your metabolic health, giving you actionable feedback.
Can environmental toxins override healthy metabolism?
Environmental toxins—such as microplastics, asbestos, and certain chemicals—can damage mitochondria and promote cancer, even in otherwise healthy individuals. While a strong metabolism offers protection, it’s not absolute. Avoiding known carcinogens and supporting your body’s detoxification through diet and exercise remain important parts of a holistic Cancer Prevention Strategy.
In summary, the metabolic approach to cancer is not a cure-all, but it offers a hopeful, actionable path forward. As research continues and new tools emerge, you have more power than ever to influence your health. Stay curious, stay informed, and remember: prevention starts with the choices you make every day.
TL;DR: Cancer may be more about ancient cell metabolism than just your genes—especially when lifestyle, diet, and mitochondrial health are considered. Understanding and adjusting these factors could offer hope for prevention and more effective therapies.
A big shoutout to The Diary Of A CEO for the enlightening content! Take a look at it here: https://youtu.be/VaVC3PAWqLk?si=fEgRiCUVR17aLjZn.